Acetic Acid (CAS 64-19-7) – Wholesale & Research Grade
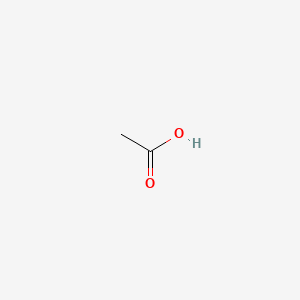
Acetic acid is a simple monocarboxylic acid containing two carbons. It has a role as a protic solvent, a food acidity regulator, an antimicrobial food preservative and a Daphnia magna metabolite. It is a conjugate acid of an acetate.
Research Context
Acetic acid, commonly known as ethanoic acid or vinegar acid, is a versatile organic compound widely utilized in various research applications. Its role as a reagent in chemical synthesis is significant, particularly in the production of acetic anhydride, acetates, and other organic derivatives. In biochemistry, acetic acid serves as a key player in metabolic pathways, acting as an intermediary in the synthesis and breakdown of fatty acids and carbohydrates.
Mechanism of Action
Acetic acid's mechanism of action is pivotal in numerous biochemical processes. It functions primarily as a proton donor, facilitating acid-base reactions. In biological systems, acetic acid can influence cellular metabolism by participating in the citric acid cycle, where it contributes to energy production. Its antimicrobial properties also make it valuable in food preservation and disinfection, as it can inhibit the growth of various pathogens.
Solubility and Storage Advice
This compound is highly soluble in water, making it accessible for various aqueous applications. When storing acetic acid, it is essential to keep it in a cool, well-ventilated area, away from heat sources and incompatible materials. Containers should be made of compatible materials, such as glass or certain plastics, to prevent degradation. Proper labeling and adherence to safety protocols are critical, as acetic acid can cause irritation upon contact with skin or eyes.
Future Research Directions
Future research on acetic acid is likely to explore its potential in green chemistry initiatives, particularly in developing sustainable methods for organic synthesis. Investigating its efficacy in bioremediation processes and as a biodegradable solvent may also yield significant environmental benefits. Additionally, ongoing studies into its role as a biofuel precursor could further enhance its value in energy applications. As researchers continue to uncover new facets of acetic acid, its application range is expected to expand, solidifying its importance in both industrial and laboratory settings.
| CAS Number | 64-19-7 |
|---|---|
| Formula | C2H4O2 |
| Mol. Weight | 60.05 g/mol |
| IUPAC Name | acetic acid |
| Grade | HPLC ≥98% |
Synthesis & Storage
Acetic Acid is supplied as a lyophilized powder to ensure stability during transit.
For long-term storage of CAS 64-19-7, we recommend maintaining at -20°C.
Researchers must reconstitute this peptide with bacteriostatic water or sterile solvent only when ready for use.
Quality Control: All batches undergo rigorous HPLC purity testing (≥98%) prior to dispatch from our USA fulfillment center.