Potassium Hydroxide (CAS 1310-58-3) – Wholesale & Research Grade
Potassium hydroxide is an alkali metal hydroxide.
Research Context
Potassium hydroxide, also known as caustic potash or lye, is a highly versatile compound widely used in various research applications across multiple disciplines. Its strong alkaline properties make it a critical reagent in organic synthesis, particularly in the production of biodiesel through transesterification processes. Additionally, it serves as a catalyst in the synthesis of soaps and detergents, facilitating the saponification reaction.
Mechanism of Action
The mechanism of action of potassium hydroxide primarily revolves around its ability to dissociate in aqueous solutions, releasing hydroxide ions. This increase in hydroxide ion concentration enhances the nucleophilicity of reactants, promoting reactions such as deprotonation and hydrolysis. In biochemical applications, it plays a vital role in pH regulation and enzyme activity modulation, making it indispensable in various laboratory protocols.
Solubility and Storage Advice
Potassium hydroxide is highly soluble in water, generating significant heat during dissolution, which necessitates careful handling. It readily absorbs moisture and carbon dioxide from the air, leading to the formation of a solid mass if not properly stored. Therefore, it is crucial to keep potassium hydroxide in airtight containers in a cool, dry place to maintain its efficacy and prevent degradation. Protective equipment, such as gloves and goggles, should always be used when handling this compound due to its corrosive nature.
Future Research Directions
Future research involving potassium hydroxide may focus on its role in green chemistry, particularly in sustainable manufacturing processes. Efforts are being directed towards optimizing its use in renewable energy applications, such as energy storage systems and biofuel production. Investigating the potential of potassium hydroxide in carbon capture technologies could also yield significant advancements in environmental chemistry, contributing to the reduction of greenhouse gas emissions. As researchers continue to explore its unique properties, potassium hydroxide remains a compound of great interest in advancing both industrial and academic fields.
| CAS Number | 1310-58-3 |
|---|---|
| Formula | HKO |
| Mol. Weight | 56.106 g/mol |
| IUPAC Name | potassium hydroxide |
| Grade | HPLC ≥98% |
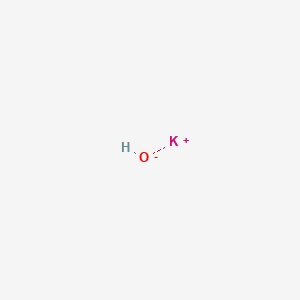
Synthesis & Storage
Potassium Hydroxide is supplied as a lyophilized powder to ensure stability during transit.
For long-term storage of CAS 1310-58-3, we recommend maintaining at -20°C.
Researchers must reconstitute this peptide with bacteriostatic water or sterile solvent only when ready for use.
Quality Control: All batches undergo rigorous HPLC purity testing (≥98%) prior to dispatch from our USA fulfillment center.